Education Links
Leaving Cert
 Maths
Maths
 French
French
 English
English
 Chemistry
Chemistry
 Physics
Physics
 Biology
Biology
 Economics
Economics
 Spanish
Spanish
 Geography
Geography
 History
History
Junior Cert
1. Endothermic and exothermic chemical reactions
Endothermic reaction: A reaction in which heat is taken in.Ex. Dissolving ammonium nitrate in water.
In an endothermic reaction heat is taken in from the surroundings and the products formed have more energy than the reactants. It is written as +
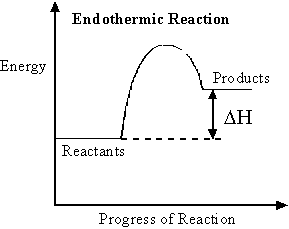
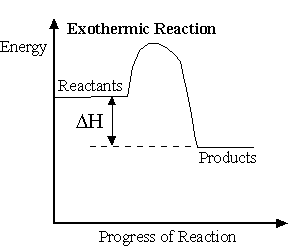
Exothermic reaction: A reaction in which heat is liberated.
Ex. Burning of coal.
In an exothermic reaction heat is lost to the surroundings and the products formed have less energy than the reactants. It is written as -
2. Definitions
Heat of Reaction: The heat change when a reaction takes place according to the given equation.Heat of Formation: The heat change when 1 mole of the compound is formed from its elements in their standard state (i.e. at 25
Heat of Combustion: The heat change when 1 mole of the substance is burned in excess oxygen.
Heat of Neutralisation: The heat change when 1 mole of
Heat of Solution: The heat change when 1 mole of the substance is dissolved in excess of solvent.
Kilogram Calorific Value: The heat liberated when 1 kg of the fuel is completely burned.
Hess's Law: The heat change for a given reaction depends only on the initial and final states of the system and is independent of the path system.
Bond Energy: The energy required to break 1 mole of covalent bonds and to separate the neutral atoms completely from each other.
3. Heat of Combustion
Experiment: To measure the heat of combustion- Place a measured volume of water in a suitable calorimeter (e.g. a metal can) and record its temperature.
- Weigh a small quantity of the substance and place it a small container (e.g. a crucible).
- Place the crucible under the calorimeter, ignite the substance and allow it to burn.
- Stir the water continuously and note the temperature when all the compound has burned.
- Calculate the quantity of heat gained by the water by using the formula:
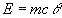
E = Energy gained by the water, m = mass of the water (kg), c = Specific heat capacity of water,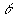 = Rise in temperature (Kelvin K)
= Rise in temperature (Kelvin K)
(To convert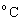 to Kelvin add 273)
to Kelvin add 273) - This is assumed to be the same amount of heat liberated by the burning substance.
- Use the molecular mass of the substance to calculate the quantity of heat which would be liberated if 1 mole of the substance was liberated.
- Heat is taken in by the calorimeter.
- Heat is taken in by the container holding the substance.
- Heat is lost to the atmosphere.
- Extra heat is supplied by the flame to ignite the substance.
- Place the burning substance as close as possible to the calorimeter.
- Lag the calorimeter around the sides.
- Put a draught shield around the apparatus.
- Stir the water continuously.
- Use an accurate thermometer.
Example 1
When 2 g of sulphur was completely burned in oxygen, the heat liberated raised the temperature of 222 g of water from 19(Specific heat capacity of water is 4200
Solution
Example 2
When 0.92 g of ethanol ((Specific heat capacity of water is 4200
Solution
Example 3 (LCH 1992)
When 1.5 g of methanol (Solution
Example 4 (LCH 1989)
When 1.0 g of pure heptane was completely burned in a suitable apparatus the rise in temperature was 5.7Solution
Example 5 (LCH 1990)
The heat of combustion of methane is -890Solution


