Education Links
Leaving Cert
 Maths
Maths
 French
French
 English
English
 Chemistry
Chemistry
 Physics
Physics
 Biology
Biology
 Economics
Economics
 Spanish
Spanish
 Geography
Geography
 History
History
Junior Cert
The rate of decay (activity) of a radioactive nuclide is directly proportional to the number of undecayed nuclei present in the sample.
Nuclear Physics can be divided into two parts:
1. Natural radioactivity and
2. Artificial Radioactivity.
We will classify the problems encountered in Radioactivity in the same way.
Natural Radioactivity Problems
These problems all involve the spontaneous decay of certain naturally occurring nuclides with the emission of an a particle, or a b particle or/and then g radiation.Type 1: Balancing Natural Nuclear Reactions.
It is important to remember that in such reactions there is only one nuclear species, on the left-hand side of the reaction, as there is no bombarding particle.
You must know:
1. Every nuclear species is written in the form where
Z = Number of protons in the nucleus (Atomic Number) and
A = Total number of particles in the nucleus (Mass Number).
2. a =
3. Conservation of charge: In all nuclear reactions the sum of the atomic number(s) before = the sum of the atomic number(s) after.
4. Conservation of nucleons: In all nuclear reactions the sum of the mass number(s) before = the sum of the mass number(s) after.
Example 1
Solution
Example 2
Solution
Type 2: Number of a and b particles emitted in successive decays.
You must know: When an a particle is emitted the mass number A of the parent nucleus decreases by 4 and its atomic number Z decreases by 2.
When a b particle is emitted the mass number A of the parent nucleus is unchanged but its atomic number Z increases by 1.
Example 3
How many a particles and b particles are emitted whenSolution
Type 3: Activity Problems
You must know
(i) Law of Radioactive Decay: The rate of decay (activity) of a radioactive nuclide is directly proportional to the number of undecayed nuclei present in the sample.
A = Activity, l = Decay constant, N = Number of undecayed atoms.
(ii) Activity is measured in decays/sec, a's/sec, disintegrations/sec etc.
The unit of activity is the Bq and is equal to one decay per second.
(iii) Avogadro's Number: A mole of every substance contains the same number of atoms equal to
Example 4
(Avogadro's No. =
Solution
Example 5
A radioactive source contains(Avogadro's No. =
Solution
Type 4: Half-Life (HL) Problems
You must know:
(i) The half-life of a radioactive isotope is the time taken for half of the nuclei in a sample of the radioactive isotope to decay.
(ii) The fraction of undecayed nuclei left after n half-lives is
(iii) The relationship between decay constant, l, and half-life,
Example 6
The half-life of a certain radioisotope is 3.8 days. What fraction is left after 19 days?
Solution
Example 7
A radioactive material has a half-life of 40 days. How long does it take seven-eighths of the material to decay.Solution
Example 8
The mass of a certain sample is found to decrease by 60% in 10 days. Find its half-life.Solution
Example 9
Calculate (a) the decay constant of(Avogadro's No. =
Solution
Artificial Radioactivity Problems
A great deal of information concerning the nucleus has been obtained by studying the effects of bombarding the atomic nucleus with missiles.Type 1: Artificial Nuclear Reactions
There are two (or more) nuclear species on the left-hand side of the equation.
You must know:
Nuclear missiles:
Example 10
Solution
Type 2: Energy Problems
You must know:
(i) Conservation of mass-energy in nuclear reactions. This means that in a nuclear reaction energy cannot be created or destroyed, it can only be converted from one form into another once mass is regarded as a form of energy.
(ii) Einstein's Mass-Energy Equation.
E: Energy released in Joules, m: Mass before - Mass after, c: Speed of light. (iii) 1 eV =
Example 11
An Irishman E.T.S. Walton (the only Irish Nobel Laureate in Physics) carried out one of the first artificial nuclear reactions.Calculate the energy released.
Constants:
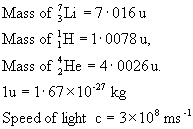
Solution
Example 12
The first nuclear fission reaction observed by Hahn and Strassmann was:Calculate the energy released.
Masses:
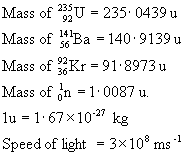
Solution


