Solution
Back
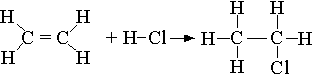
(ii) Calculate the total energy required to break all the bonds in the reactant molecules.
Breaking 1 mole C=C: 1 (+ 612) = + 612 kJ
Breaking 4 mole C-H: 4 (412) = + 1648 kJ
Breaking 1 mole H-Cl: 1 (431) = + 431 kJ
Total energy required = + 2691 kJ
(iii) Calculate the total energy released when bonds in product molecule are formed.
Forming 1 mole C-C: 1(- 348) = - 348 kJ
Forming 5 moles C-H: 5(- 412) = - 2060 kJ
Forming 1 mole C-Cl: 1(-x) = - x kJ
Total energy released = - 2408 - x
(iv) Equate the sum of (ii) and (iii) with the heat of reaction (heat of reaction given in question = - 55 kJ)
(i) Write the equation showing the individual bonds.
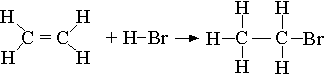
(ii) Energy required:
Breaking 1 mole C=C: 1(+ 612) = + 612 kJ
Breaking 4 mole C-H: 4(412) = + 1648 kJ
Breaking 1 mole H-Br: 1(366) = + 366 kJ
Total energy required = + 2626 kJ
(iii) Energy release
Forming 1 mole C-C: 1(- 348) = - 348 kJ
Forming 5 moles C-H: 5(- 412) = - 2060 kJ
Forming 1 mole C-Br: 1(-x) = -x kJ
Total energy released= -2408 - x
(iv) Equate
2626 - 2408 - x = - 58